Regulation
Alberta Spreafico, managing director of digital health and innovation at Healthware, shares how the report can guide companies' go-to-market strategies for entering the European market.
The company was also the first to receive emergency use authorization for its home COVID-19 test in 2021.
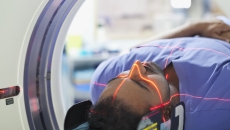
The company's Precision DL speeds up screening time, enhances the image quality of PET/CT scans and improves small lesion detection.
The company's iPredict System allows providers to detect age-related macular degeneration in the clinical setting.
Andy Molnar, CEO of the Digital Therapeutics Alliance, discusses the market for digital therapeutics after Pear Therapeutics declared bankruptcy.
The company's ANNE One platform allows for wireless monitoring of neonates and infants' skin and body temperature, heart and respiratory rates, and more.
The feature monitors heart rhythms suggestive of atrial fibrillation through the Galaxy Watch.
The startup plans to file with the FDA to add other monitoring abilities and to introduce the product later this year.
At HIMSS23, Jodi Daniel, managing director of Crowell Health Solutions, discussed the evolving policy landscape for telehealth, remote patient monitoring and other digital health technologies as the public health emergency comes to a close.
A federal jury found three former executives guilty of defrauding investors and clients, largely pharma companies.