Regulation
The company is also recruiting a CEO in the US.
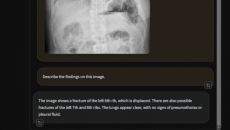
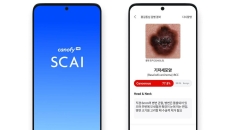
Also, See-Mode has received the first US FDA approval for a thyroid ultrasound analysis AI solution.
Digital Therapeutics Alliance CEO joins FDA Digital Health Advisory Committee and more digital heal…
Arrive AI appoints a former Elevance Health executive to its board, and CareAbout Health hires a seasoned digital health executive to be its new chief technology officer.
The Act is the world's first major law for AI implementation and provides regulations for high-risk applications of the technology, including its use in healthcare.
The t:slim X2 Pump is compatible with Dexcom G7 and G6 continuous glucose monitoring systems and was cleared for sale by Health Canada.
LifeSemantics plans to commercialise its mobile app-based AI screening solution by yearend.
The company's Dream Sock, which received FDA 510(k) clearance in November, gathers real-time health readings, including a baby's sleep trends and pulse rate.
The Agency has qualified Apple's atrial fibrillation history feature as a medical device development tool, allowing it to be used in clinical trials.
The Agency alleges the company's CT and ultrasonography systems are noncompliant with good manufacturing requirements.
They had been designated as innovative medical devices.