FDA
The FDA clarifies its interpretation of "more effective," and indicates it will consider improved accessibility of a device during the Breakthrough designation process.
The FDA's Oncology Center of Excellence will use Aetion's Evidence Platform to find and analyze sources of real-world data that could be used for research.
The Class I recall of the DASH system comes days after the company issued a voluntary urgent medical device "correction" for its Omnipod 5 controller.
When determining approval for Breakthrough Device designation, the FDA will consider whether the device addresses present health inequities.
The company will use the funding to support the commercial expansion of its product.
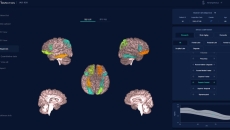
It measures the cortical thickness in a brain scan to provide a patient's level of brain ageing.
It has been approved as a prescription-only software-as-a-medical device in the US.
Also, I-MED will expand its deployment of 4DMedical's lung imaging tool across its network in Australia.
The stock took a tumble in October following the letter.
The news comes about a week after AppliedVR announced it raised $36 million in Series B funding.